An Everyday DNA blog article
By: Sarah Sharman, PhD, Science writer
Illustrated by: Cathleen Shaw
 Katie suffered neurological symptoms for three years and had not received a diagnosis despite visiting many specialists. Her primary physician recommended that Katie undergo clinical genome sequencing to determine if there was a genetic change that could explain her symptoms.
Katie suffered neurological symptoms for three years and had not received a diagnosis despite visiting many specialists. Her primary physician recommended that Katie undergo clinical genome sequencing to determine if there was a genetic change that could explain her symptoms.
When Katie received her testing report, it identified a genetic change likely responsible for her neurological symptoms. The report also identified a change in the BRCA1 gene that increases Katie’s risk of breast, ovarian, and other cancers. Why did Katie receive information about a disease for which she was not showing symptoms?
Secondary findings provide information about genetic changes unrelated to the primary reason for the genetic test, in this case, Katie’s neurological symptoms. Let’s learn more about secondary findings, what genes and diseases are included in the secondary findings list, and how genes make it on the list in the first place.
What are secondary findings?
Suppose a patient’s symptoms are consistent with a genetic condition or disease. In that case, their physician might order a gene panel that looks at a few genes most likely to be associated with the disease. However, suppose the patient’s symptoms do not fit one specific genetic disease or group of diseases. In that case, the physician might order a broader type of genetic test such as exome or genome sequencing. Clinical exome sequencing looks at the protein-coding parts of DNA in a person’s genome, while clinical genome sequencing looks at all of the DNA in a person’s genome.
Clinical genome and exome sequencing can help diagnose the cause of a person’s symptoms. In addition to information relevant to the question being asked about the cause of an individual’s symptoms, these tests also identify many more genetic changes, or variants, beyond the potential disease-causing change. The vast majority of these changes are likely to be harmless or benign. However, sometimes the variants are associated with an increased risk of developing a known condition other than the one for which the testing was originally performed.
The American College of Medical Genetics and Genomics (ACMG) is a nationally recognized organization of experts in the field of genomics who are committed to improving the practice of medical genetics. As part of these efforts, ACMG members maintain recommendations for reporting secondary findings in clinical exome or genome testing. The current recommendations establish a list of 73 genes, “the ACMG 73,” that clinical testing laboratories should analyze for secondary findings during exome or genome sequencing.
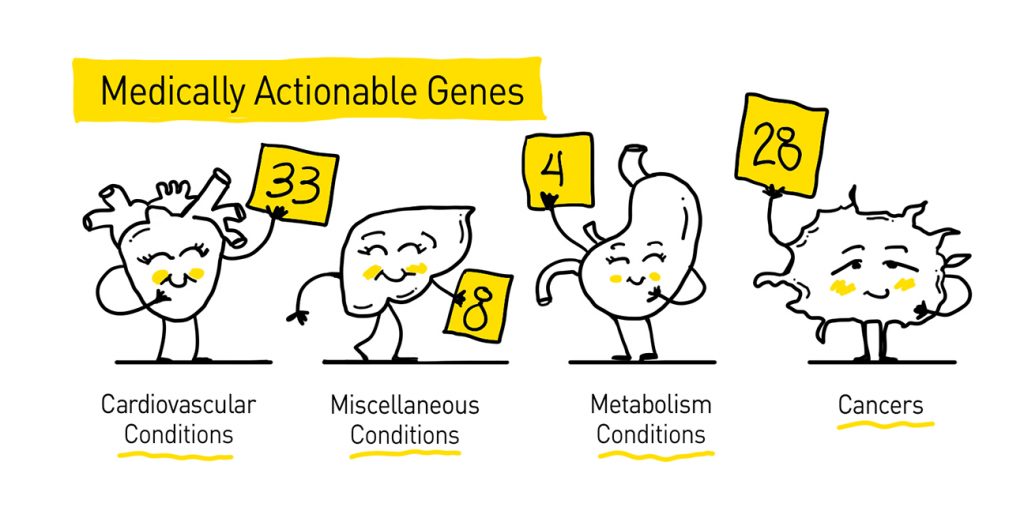 The ACMG breaks its list of genes down into four categories: genes involved in hereditary cancers (28 genes), genes of inborn errors of metabolism (4 genes), cardiovascular conditions (33 genes), and miscellaneous conditions (8 genes). Disorders on the ACMG 73 list include hereditary cancer syndromes, connective tissue disorders, cardiac disorders, metabolic disorders, malignant hyperthermia, and familial hypercholesterolemia.
The ACMG breaks its list of genes down into four categories: genes involved in hereditary cancers (28 genes), genes of inborn errors of metabolism (4 genes), cardiovascular conditions (33 genes), and miscellaneous conditions (8 genes). Disorders on the ACMG 73 list include hereditary cancer syndromes, connective tissue disorders, cardiac disorders, metabolic disorders, malignant hyperthermia, and familial hypercholesterolemia.
Secondary findings offer the chance to identify and mitigate diseases that may otherwise be unrecognized in an individual. All genes included in the ACMG 73 list are medically actionable, meaning the genes are related to diseases or conditions with known medical interventions.
One example of a secondary finding gene is MSH2, which is associated with Lynch syndrome. Having Lynch syndrome increases a person’s chance of developing colorectal, endometrial/uterine, stomach, ovarian, and small bowel cancers. Individuals with harmful changes in MSH2 or other Lynch-associated genes can begin early detection and prevention methods such as earlier and more frequent colonoscopies and endoscopies, preventative surgeries, blood tests and urinalysis to look for disease biomarkers, and more.
How do genes make it on the secondary findings list?
There are about 20,000 genes in the human genome. So, why does the ACMG recommend reporting changes in only 73 genes as secondary findings? The ACMG sets rigorous standards for gene-disease interactions to be included on the secondary findings list. Telling a person they have a higher-than-average chance of one day developing a disease is a serious matter that experts want to ensure is as accurate as possible.
The ACMG takes nominations yearly from committee members for new gene-disease interactions to be included in the secondary finding list. First and foremost, the gene should be associated with a disease or condition that is severe and actionable. Scientific studies and clinical data should support the connection between the gene and associated disease. In addition, the possibility of early diagnosis, a reliable clinical genetic test, and effective interventions or treatments should be available for each disease.
The ACMG also selects genes based on the technical feasibility of detecting variants in them, only including genes in which disease-causing variants can be easily identified from sequencing data without an undue analytic burden. Diseases are not included if the timing of the diagnosis is not critical for treatment or if the primary intervention for the disease is a lifestyle change like quitting smoking.
So, you received a secondary finding. What do you do now?
Individuals receiving genomic testing should talk with their healthcare provider to learn whether they have to opt-in to receive secondary findings or are automatically included in the testing. Pre-testing genetic counseling is strongly encouraged for anyone undergoing genetic testing. Genetic counselors, like those at Smith Family Clinic, LLC, located on HudsonAlpha Institute for Biotechnology’s campus, are trained to help individuals make informed decisions regarding genetic testing, including whether or not they wish to learn of any secondary findings. They are also trained to return genetic testing results.

Although Katie’s primary physician originally suggested genome sequencing, her test was ordered through a geneticist’s office, where a genetic counselor could participate in her care. Her pre-testing counseling included an in-depth conversation with a genetic counselor about what secondary findings would be returned and expectations of actions that Katie could take should she receive secondary findings.
When returning Katie’s results to her, the genetic counselor first discussed the genetic finding that likely explains her neurological symptoms. Next, the genetic counselor explained her secondary finding of a change in BRCA1 that increases Katie’s chance of one day developing breast, ovarian, or other cancers.
The genetic counselor referred Katie to a cancer genetic specialist who can prepare a personalized plan to lower her cancer risk. The plan includes early and more frequent screening tests as well as the option for preventative surgeries. Although it was not the original reason for her genetic testing, Katie’s secondary findings will allow her the opportunity to be more proactive in her health and reduce her chance of developing cancer in the future.


