An Everyday DNA blog article
Written by: Sarah Sharman, PhD, Science writer
Illustrations by: Cathleen Shaw
As scientists learn more about the human genome, it is becoming clear that many diseases are caused by changes in our genetic material. Some diseases that are present at birth are caused by genetic changes inherited from an individual’s parents, while others occur from new (de novo) changes not present in the parents’ DNA. Other diseases that show up later in life are often caused by acquired changes in a gene or group of genes that occur randomly during the person’s life. Such changes are not inherited but occur either randomly or due to environmental exposure, like cigarette smoke.
Advances in genetic sequencing have connected many genetic changes to human diseases. The cause of many diseases is complex, being influenced by changes in many genes in combination with lifestyle and environmental factors. However, thousands of diseases are caused by changes in one gene that is enough to cause the disease entirely on its own. Such diseases are called single-gene diseases or Mendelian diseases. Let’s dive in and learn about single-gene diseases. But first, we need to understand a little bit about the human genome and its impact on health and disease.
What is DNA?
DNA, short for deoxyribonucleic acid, is a long molecule that contains your unique genetic code. Nearly every cell in your body contains DNA within a structure called a nucleus. For those of you wondering why I said nearly every cell, it is because mature red blood cells and cornified cells in your skin, hair, and nails do not contain nuclei to store DNA but that is a story for another blog post.
If you could remove a strand of DNA from the nucleus of a cell and stretch it out, it would be 6.5 feet long. Imagine trying to fit that much DNA into a microscopic nucleus. Sounds impossible, right? Luckily, DNA is organized into tightly wound coils called chromosomes that are compact enough to fit into the nucleus. 
Each chromosome contains many genes, which are small sections of DNA that carry the instructions for our individual traits and characteristics. The human genome contains an estimated 30,000 genes which encode for proteins that influence things like the immune system, hormone production, and hair color.
Genes can acquire changes in their DNA sequence either randomly or through environmental exposure. Different versions of the same gene are called alleles. The alleles of the same gene still code for the same trait, but they differ in how the trait is expressed. Many gene changes do not cause problems but some are known to cause disease.
How are single-gene diseases inherited?
Now that we understand DNA and its role in health and disease, let’s discuss where your genes come from. Most of the cells in your body normally contain 46 chromosomes that are divided into 23 pairs. Chromosome pairs one to twenty-two are called autosomes. The 23rd pair of chromosomes are called sex chromosomes and they determine if a person is genetically male (XY) or female (XX). Children randomly inherit one copy of each chromosome from their mom and the other from their dad.
Genetic diseases can be described based on the type of chromosome that contains the gene change. If the gene is on an autosome, the disorder is called an autosomal condition. If the gene is on one of the sex chromosomes, the disorder is called sex-linked.
Single-gene diseases are caused by changes in the DNA sequence of a specific gene. Because they are caused by specific gene changes, single-gene disorders may run in families in a predictable pattern. The chance of a parent passing a genetic disease to their children depends heavily on the inheritance pattern of the disease.
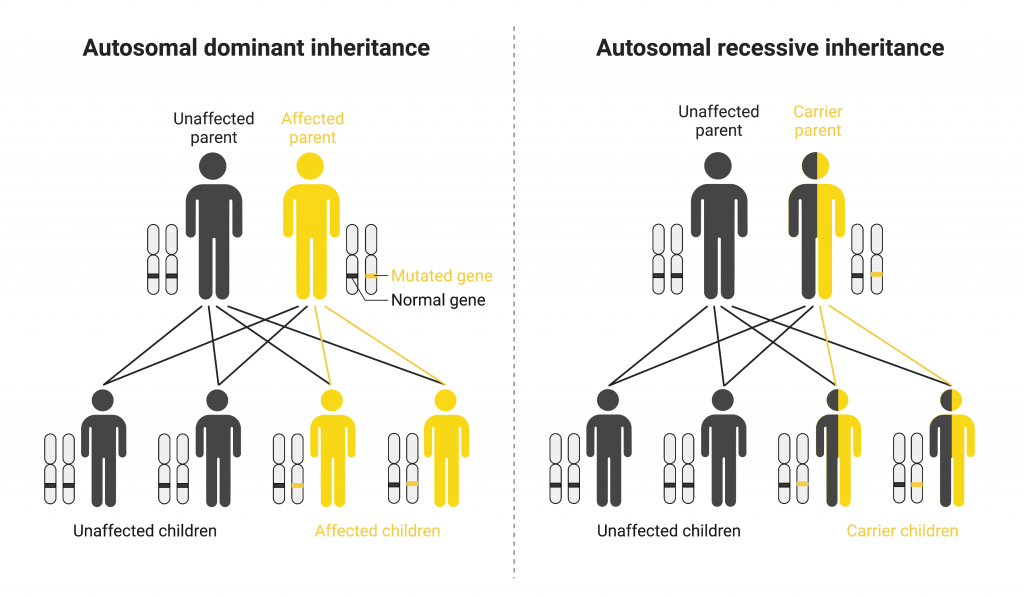
In addition to being autosomal or sex-linked, diseases are also classified based on how they run in families. Diseases caused by one copy of a gene having a disease-causing DNA change are called dominant diseases. Despite a second unchanged copy of the gene, having one copy of the gene change is sufficient to cause disease symptoms. If one parent has a dominant genetic disease, each of their children has a 50 percent chance of inheriting the gene change and therefore the disease. Examples of autosomal dominant diseases include Huntington’s disease and Marfan syndrome.
Recessive diseases on the other hand require that both copies of the gene contain a disease-causing DNA change in order for the individual to have the disease. If both parents are carriers with one copy of the gene change, each of their children would have a 25 percent chance of inheriting two copies of the gene change and having the disease. Carriers of recessive diseases can pass the disease-causing change onto their children even though they do not have the disease themselves. Examples of autosomal recessive diseases include cystic fibrosis, sickle cell anemia, and phenylketonuria.
X-linked diseases are caused by changes in genes on the X chromosome. In males, who only have one X chromosome, a change in the X chromosome is sufficient to cause disease. For females, if the condition is dominant, inheriting one X chromosome containing the gene change is also sufficient to cause disease. However, if the condition is recessive, one copy of the disease-causing genetic change could cause mild disease or no disease at all in females. One feature of X-linked inheritance is that fathers cannot pass X-linked traits to their sons since they inherit the Y chromosome from their fathers. Examples of X-linked diseases include Fragile X syndrome, Duchenne muscular dystrophy, some types of colorblindness, and hemophilia A.

Similar to X-linked diseases, Y-linked diseases are caused by changes in genes on the Y chromosome. Because the Y chromosome is so much smaller than the X chromosome, it contains far fewer genes. Accordingly, there are far fewer Y-linked diseases. Examples of Y-linked diseases include Y chromosome infertility and some cases of Swyer syndrome.
How are single-gene diseases diagnosed?
Discoveries in genetic research, made possible in part by DNA sequencing technology, have unearthed tremendous opportunities in the diagnosis, detection, and treatment of countless genetic diseases. To date, the genetic basis for thousands of single-gene diseases has been discovered, leading to growing diagnostic potential using genetic testing.
Genetic testing involves analyzing an individual’s DNA for specific changes that are correlated with a certain disease. It is one of several tools used to diagnose genetic diseases. During these tests, individuals provide a DNA sample (usually in the form of saliva or blood) that is then analyzed for genetic changes. Genetic tests are not limited to a single gene, some genetic tests called gene panels analyze multiple genes at once. There are currently genetic tests available for many conditions.
 For individuals with undiagnosed disease symptoms or a family history of a genetic disease, genetic testing plays a vital role in diagnosing disease, determining an individual’s risk of developing certain diseases, and can also be useful in medical treatment planning. Additionally, if someone knows that a genetic disorder runs in their family, they can have a genetic test to understand their risk of developing the disease as well as their risk of passing the disease on to children.
For individuals with undiagnosed disease symptoms or a family history of a genetic disease, genetic testing plays a vital role in diagnosing disease, determining an individual’s risk of developing certain diseases, and can also be useful in medical treatment planning. Additionally, if someone knows that a genetic disorder runs in their family, they can have a genetic test to understand their risk of developing the disease as well as their risk of passing the disease on to children.
Genome sequencing recently emerged as another critical tool in the diagnosis of genetic diseases. This type of genetic test looks at an individual’s entire genetic code, rather than focusing on just a selected set of genes and gene changes. Genome sequencing has led to the discovery of new disease-causing genetic changes and also facilitates diagnoses for individuals with rare genetic conditions that have gone undiagnosed using other methods.
The Smith Family Clinic for Genomic Medicine is a stand-alone medical office residing on the HudsonAlpha Institute for Biotechnology campus that focuses on the diagnosis of rare genetic diseases using genomics. The clinic, led by board-certified genetic counselor Kelly East, MS, CGC, aims to diagnose patients with undiagnosed and misdiagnosed diseases. A unique part of the clinic is that they integrate genome sequencing into the routine practice of genetics, something that not many clinics are currently doing.
While genetic testing and specifically genome sequencing are game-changers for diagnosing genetic conditions, they can be expensive and are not always covered by insurance. Leaders at HudsonAlpha and the Smith Family Clinic aim to give every patient access to cutting-edge genomic medicine. The Hero Fund, established by an anonymous donation to the HudsonAlpha Foundation, helps patients at the Smith Family Clinic who need, but cannot afford, genome sequencing. To learn more about the Hero Fund, or to donate to the fund, visit https://smithfamilyclinic.org/hero/.