An Everyday DNA blog article
Written by: Sarah Sharman, PhD, Science writer
Illustrated by: Cathleen Shaw

Anyone who became a “plant lady” during the pandemic like me knows that house plants have several needs in order to grow and thrive. Some of my plants are content in a shady corner with a drink of water every few weeks. My moodier plant children, however, will shrivel up and turn yellow if even a drop of unfiltered water gets on their soil. Determining the right conditions for each plant can feel like an impossible feat sometimes.
In addition to water and ample sunlight, plants also require several nutrients such as nitrogen, phosphorus, and potassium to survive. They use these nutrients to carry out many important biological functions like growth, germination, fighting off diseases and pests, and reproducing. Nitrogen is king among the primary nutrients required by plants to survive and flourish.
While houseplants bring us joy (and frustration), there is another group of plants that are very important for life on Earth, crop plants. Let’s learn about the many ways that plants can get their nitrogen fix and how scientists are helping develop sustainable methods to make sure crop plants receive the nitrogen they need to continue feeding our growing population.
What’s the big hype about nitrogen, anyways?
Nitrogen is a colorless, odorless element that is essential to life on Earth. It is in the air we breathe, the water we drink, and the soil under our feet. In fact, our air is about 78 percent nitrogen, making it the most abundant element in Earth’s atmosphere.
Nitrogen is a key component in nucleic acids, like DNA and RNA, which are the most important biological molecules for most organisms. It is also a major part of amino acids, which are the building blocks of proteins. Normal cell growth, cell replacement, and tissue repair also require nitrogen for the production of new cells.
In plants, nitrogen is essential for growth, survival, and photosynthesis. Without it, plants will turn yellow and stop growing. Plants use nitrogen to produce proteins, enzymes, amino acids, and nucleotides, all of which people absorb when they eat plants.
Although the air we breathe contains large quantities of nitrogen, it exists in a form that our bodies cannot use. The two nitrogen atoms in atmospheric nitrogen (N2) are tightly bound with a triple bond so our bodies cannot break them down into individual molecules. Because of this, humans must absorb nitrogen in the form of ammonia (NH3) through their diets, predominantly from plants but also from meat.
So if humans get their nitrogen from plants, that begs the question: where do plants get their nitrogen? Like humans, plants cannot use atmospheric nitrogen directly. Instead, they take up usable forms of nitrogen like nitrates and ammonia from water and soil. A process called the nitrogen cycle, which we will discuss in detail below, converts atmospheric nitrogen into more usable forms.
Where do plants get nitrogen from?
The nitrogen cycle is a repeating cycle of processes through which nitrogen moves and changes form from the atmosphere to soil to living organisms and back into the atmosphere. There are five steps to the nitrogen cycle: fixation, mineralization, nitrification, immobilization, and denitrification.
During nitrogen fixation, nitrogen moves from the atmosphere to the soil and is converted (fixed) into forms that plants can absorb through their roots. Nitrogen can be fixed in the atmosphere by lightning which provides energy for nitrogen gas to react with oxygen and produce nitrogen oxides. These forms then enter the soil through rain or snow.
Most nitrogen fixation however occurs naturally in the soil through bacteria that use an enzyme to convert nitrogen gas to ammonia. Some bacteria live on plant roots and gain energy from the plant. In return, they fix nitrogen that can be carried up the roots to the plant.
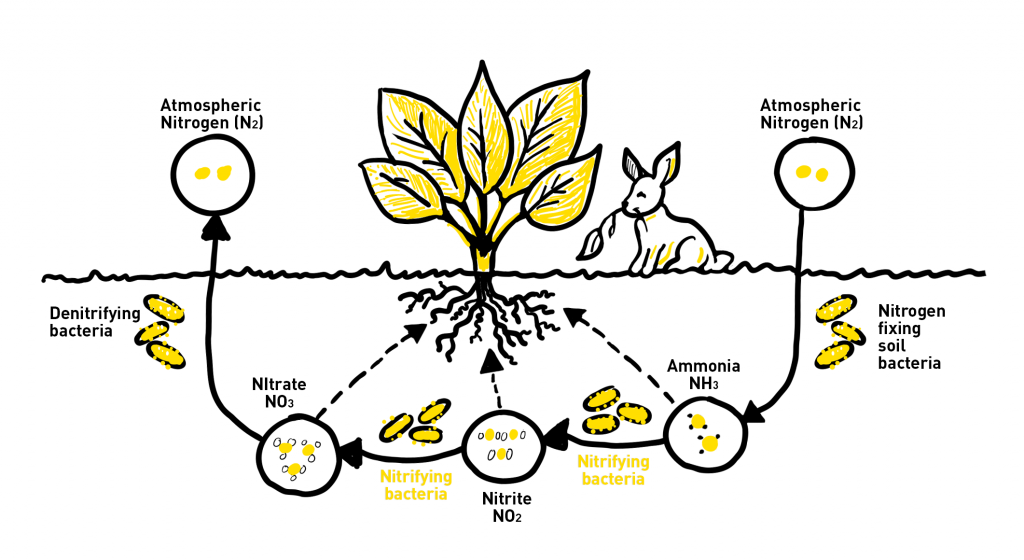 For plants that do not obtain enough nitrogen from fixation, a process called mineralization is important. Microbes in the soil feed on decomposing plant and animal material and produce ammonia and other forms of nitrogen that can be used by plants.
For plants that do not obtain enough nitrogen from fixation, a process called mineralization is important. Microbes in the soil feed on decomposing plant and animal material and produce ammonia and other forms of nitrogen that can be used by plants.
During nitrification, ammonia produced during nitrogen fixation is converted further into more usable forms of nitrogen called nitrite and nitrate, which the plant roots absorb as nutrients. Soil microbes don’t just convert nitrogen, they also store it themselves, in the process called immobilization. In the final step of the nitrogen cycle, denitrifying bacteria convert ammonia back into atmospheric nitrogen, which is released into the atmosphere where the nitrogen cycle starts again.
When plants die, the nitrogen within them returns to the soil, and new plants use it to grow. In modern agriculture, we interrupt this cycle by harvesting the plants so that they cannot contribute their nutrients back to the soil. In order to have a successful harvest the next growing season, farmers must put the nutrients back into the soil. In early farming practices, natural fertilizers like compost or manure were used. The introduction of synthetic fertilizers in the early 1900s was a game-changer for modern agriculture. Farmers can use nitrogen fertilizers to replace nutrients lost from the soil and boost their crop yield each season.
How are scientists helping plants sustainably obtain nitrogen?
While fertilizers are fundamental in feeding our growing population, we cannot keep solely relying on them. Synthetic fertilizers are expensive and overuse of fertilizers can lead to environmental problems like runoff into water systems and the release of greenhouse gasses. Providing farmers with other alternatives to use in addition to fertilizers will give them a more well-rounded toolbox with which to feed and nourish their crop plants.
So what is a viable alternative to synthetic fertilizers? Let’s revisit the fact that some plants have bacterial friends living in their root systems, bathing them in nitrogen. Legumes such as clover, soybean, and more, developed close relationships with nitrogen-fixing bacteria over time. The roots of such plants contain protective structures called nodules in which the bacteria can live. Planting nitrogen-fixing crops as cover crops between seasons can replenish the nitrogen stores in the soil for the next round of crops.
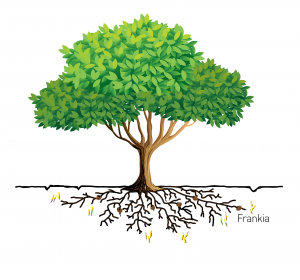
While much is known about nitrogen fixation in legumes, there is not much information available for non-legume nitrogen fixation. At the HudsonAlpha Institute for Biotechnology, researchers are looking at the symbiotic relationship between the Southern wax myrtle tree (Morella cerifera) and its bacterial partner Frankia. Laramie Aközbek (nee Smith), a first-year Auburn University PhD student in the lab of Auburn University professor and HudsonAlpha faculty investigator Alex Harkess, PhD, recently received a three-year National Science Foundation Graduate Research Student fellowship to fund the project.
The team will sequence the genomes of both M. cerifera and Frankia to determine if there are genes responsible for nitrogen fixation that have been conserved across species during evolution. The genomic information uncovered during the project will also give Aközbek and the team in the Harkess lab insight into the evolution of nitrogen fixation across plant species. A deeper understanding of nitrogen fixation could one day provide farmers with an additional tool in their arsenal to help grow healthy, robust crop plants and feed our growing population.


