Gene therapy for sickle cell disease shows promise
 Many human diseases are caused by mutations in a single gene of an individual’s DNA. For decades, scientists and clinicians have searched for technologies to correct the effect of those genetic mis-spellings. One approach falls under the broad heading of gene therapy. This includes replacing a gene that isn’t working properly, “turning on” a gene that is not working at all, adding a “good” gene into a person who has a disease, or blocking a gene that is causing a problem.
Many human diseases are caused by mutations in a single gene of an individual’s DNA. For decades, scientists and clinicians have searched for technologies to correct the effect of those genetic mis-spellings. One approach falls under the broad heading of gene therapy. This includes replacing a gene that isn’t working properly, “turning on” a gene that is not working at all, adding a “good” gene into a person who has a disease, or blocking a gene that is causing a problem.
Gene therapy holds promise for treating a wide range of diseases, but historically it has seen more failures than successes. However, within the last decade the field has begun stacking up some impressive wins. One experimental form of gene therapy directly changes a patient’s DNA through CRISPR-based gene editing. Recently, several patients with a blood disorder called sickle cell disease have found relief from their excruciating symptoms thanks to a clinical trial using this type of gene therapy.
What is sickle cell disease?
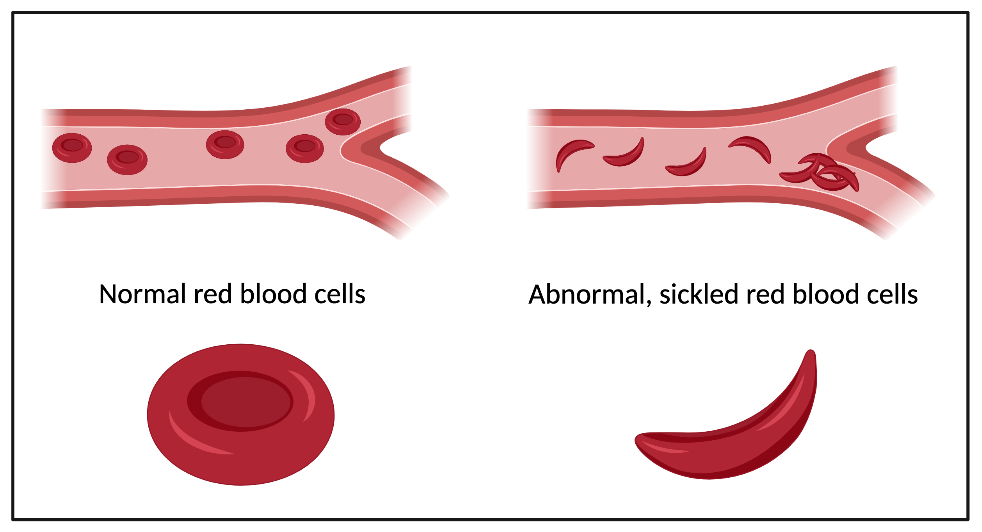
Sickle cell disease is a group of inherited blood disorders that affects about 6 million people worldwide. The most common type of sickle cell disease is sickle cell anemia. In the United States, the disease disproportionately affects people of African American descent, although people with sickle cell disease also come from Hispanic, Middle Eastern, Asian Indian and southern European backgrounds. Sickle cell disease is caused by a mutation in the gene that codes for one part of a multi-protein complex called hemoglobin.
In healthy red blood cells, hemoglobin binds oxygen so the cells can carry it from the lungs to tissues throughout the body. In sickle cell patients, if hemoglobin is not actively binding to an oxygen molecule, it can stick to other hemoglobin proteins, causing large clumps. These hemoglobin masses cause the red blood cells to become rigid, sticky, and misshapen from their healthy doughnut shape into a shape like the letter “C” (it is called sickle cell disease because the C-shape of the red blood cells are similar to the farm tool called a “sickle”).
As the sickled cells travel through small blood vessels, they can get stuck and clog up blood flow, causing what are known as “pain crises”. Pain is the most common symptom of sickle cell disease. Mutated hemoglobin also damages red blood cells by poking through their cell walls, reducing their life span from 120 days to fewer than 20 days. If the body cannot replace the red blood cells fast enough, the patient could suffer from anemia (low red blood cell count). Other symptoms of the disease include infection and organ damage.
The only known cure for sickle cell disease is bone marrow transplantation which is expensive and carries significant risks. There are only four approved medications for the treatment of sickle cell disease. However, in many of the areas in which sickle cell disease is highly prevalent, these drugs are not affordable or widely available. Without a good treatment for the disease itself, doctors must treat the patients symptomatically with medications and treatments for pain, anemia, and infection.
The only known cure for sickle cell disease is bone marrow transplantation which is expensive and carries significant risks. There are only four approved medications for the treatment of sickle cell disease. However, in many of the areas in which sickle cell disease is highly prevalent, these drugs are not affordable or widely available. Without a good treatment for the disease itself, doctors must treat the patients symptomatically with medications and treatments for pain, anemia, and infection.
Gene editing to treat sickle cell disease
A new treatment for sickle cell disease is in early clinical trials but has reported promising results. The treatment uses gene editing technology (CRISPR-Cas9) on the patient’s own stem cells to treat the disease. CRISPR-Cas9 is an enzyme that can be targeted to make specific cuts in a cell’s DNA. In the case of the sickle cell disease treatment, CRISPR is used to edit stem cells and allow them to produce a slightly different type of hemoglobin called fetal hemoglobin.
While babies are in the womb, their red blood cells have fetal hemoglobin which binds tightly to oxygen, allowing it to compete successfully with its mother’s hemoglobin for oxygen. After birth, the red blood cells switch from producing fetal hemoglobin to adult hemoglobin. Only adult hemoglobin carries the sickle mutation which is why babies in utero do not have sickle cell crises. 
Using gene editing, scientists are trying to reawaken fetal hemoglobin production by disabling the gene that turns it off. To do this, bone marrow stem cells are removed from the individual with sickle cell disease. CRISPR is used to disable the gene that turns off fetal hemoglobin production in those cells. The edited cells are transfused back to the patient and the now active fetal hemoglobin can carry oxygen throughout the body. By restoring production of fetal hemoglobin, researchers hope it will compensate for the defective hemoglobin produced by sickle cell patients.
Success stories
In 2019, Victoria Gray became the first person in the United States with a genetic disorder to be treated with CRISPR. Gray, who has sickle cell disease, was the first participant to receive Vertex Pharmaceuticals and CRISPR Therapeutics CRISPR-based sickle cell treatment.
One year after her treatment, Gray has not had any severe pain attacks and has not required any emergency room treatments, hospitalizations, or blood transfusions. Nearly every red blood cell in her body produces some level of fetal hemoglobin and about 46% of her total hemoglobin is fetal.
In their initial paper, the researchers also reported that two other study participants with a related condition called beta thalassemia are benefiting from a similar type of treatment. A recent press release from Vertex Pharmaceuticals and CRISPR Therapeutics highlights favorable results on seven sickle cell and fifteen thalassemia patients. Although these treatments are still in early trials, the results look promising and could one day bring relief to other individuals suffering from these types of blood-based genetic disease.
To schedule a media interview with Dr. Neil Lamb or to invite him to speak at an event or conference, please contact Lara Burhenn by email at lburhenn@hudsonalpha.org or by phone: Office (256) 327-5216 | Cell (256) 937-8210