An Everyday DNA blog article
Written by: Sarah Sharman, PhD, Science writer
Illustrated by: Cathleen Shaw
I like to think that overall, I am a pretty healthy person—my blood pressure is within the normal range for someone my age, I exercise regularly, and I eat my fruits and vegetables (with the occasional ice cream sandwich here or there). However, when my doctor reviewed my family history and suggested it might be informative to have genetic testing to look at cancer predisposition genes, I agreed.
There are many genetic products on the market, ranging from tests to learn your ancestry to tests that inform you of potential health risks in your future. Most of the tests (like the one I had done) look at only a snapshot of a person’s genome and report on a small list of gene variants known to be associated with a disease.
However, clinical genome sequencing, which takes a detailed picture of all of your DNA, is being used more often to identify gene variants related to disease risk and other health parameters in seemingly healthy individuals. Let’s discuss elective genome testing and what you can learn from it.
What is elective genome sequencing?
Whole genome sequencing is a genetic test that looks at a person’s entire genetic code, or genome. The human genome is made up of nearly 6.4 billion DNA base pairs (A, T, C and G) that we inherit from our parents. Our genome carries all of the instructions our bodies need to function and makes us who we are.
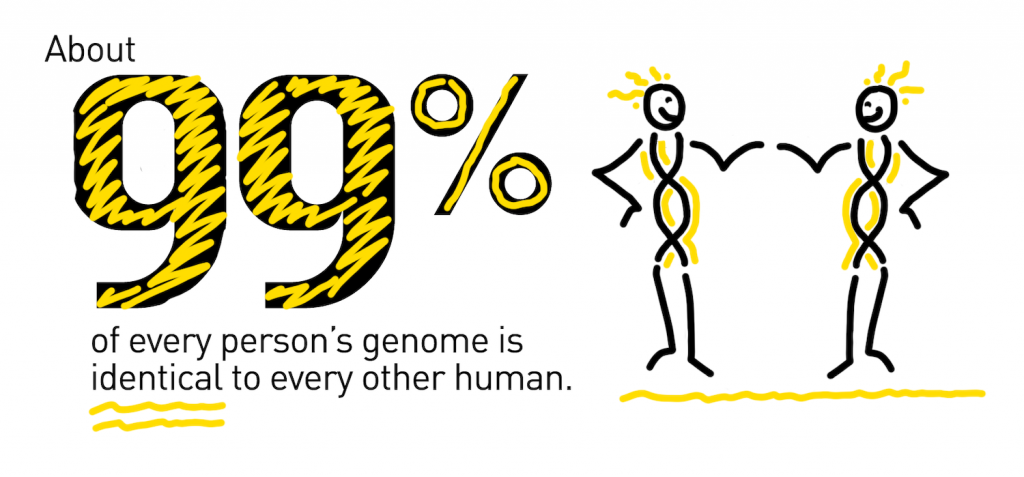
About 99 percent of every person’s genome is identical to every other human. The one percent that is unique contains millions of variants, most of which have little effect on our health. However, scientists have discovered hundreds of gene variants that cause or increase a person’s risk of developing disease.
Until recently, clinical genome sequencing has been largely used to help diagnose rare and undiagnosed diseases. However, the falling price of DNA sequencing, combined with the growing number of DNA variants that have been found to significantly impact disease risk, has led companies and genomics institutions to begin offering elective clinical genome sequencing to individuals without apparent disease.
What can you learn from your genome?
The surging popularity of direct-to-consumer DNA testing kits sparked people’s curiosity about how their DNA affects their ancestry and health. However, these kits only report on a small subset of a person’s genome. Clinical genome sequencing tests offer a much deeper look into a person’s DNA by looking at their entire genetic code, not just a subset of variants like in other tests.
Many people elect to have their genome sequenced to gain a better understanding of current medical problems, gain information about future disease risk to guide preventative healthcare decisions, fill in gaps in family history, or provide information to relatives and future generations.
Current medical concerns
During elective clinical genome sequencing, information gleaned from a person’s genome is combined with their medical and family history to provide a report with primary and secondary findings. Primary findings include variants that could explain an aspect of the person’s medical or family history. For example, if the person has high cholesterol, it could be explained by a variant in the LDLR gene.
Secondary findings are variants that do not appear to be associated with the person’s medical or family history at the time of the testing but are clinically relevant. For example, a pathogenic BRCA1 variant in a person with no known personal or family history of breast or ovarian cancer would be reported to the person so they could take appropriate screening measures. It does not mean that the person will develop the disease, it just increases the risk that they could one day.
Future disease risk
Clinical genome sequencing analyzes over 20,000 genes in the genome and reports on variants associated with the risk of developing more than 6,000 medical conditions, including hundreds of actionable diseases for which treatments or interventions are available. The American College of Medical Genetics and Genomics currently recognizes more than 70 genes associated with treatable diseases, including hereditary forms of cardiovascular disease or cancer risk, as well as several other rare genetic diseases.
Participants in elective clinical genome sequencing can also decide to receive information about adult-onset diseases, like Alzheimer disease and Huntington disease, that do not currently have a treatment available. In addition, common health conditions like diabetes or high blood pressure have genetic risk factors that can be identified through genome sequencing.
Learning about disease risk before symptoms of disease appear allows a person to make lifestyle changes or initiate preventative care measures. People with increased risk can work with their physician to prevent the onset of disease or detect the disease early and develop an appropriate treatment plan. Knowledge of gene variants that predispose a person to disease can also help couples make informed decisions when planning a family.
Pharmacogenomic information
Medications have saved many lives and cured countless diseases throughout time. However, all drugs do not always work for all people. Some people have genetic variants that affect the rate their body breaks down a certain drug.
If a drug is broken down too quickly, it is eliminated from the patient’s body before it can work at effective levels. Alternatively, if a drug is broken down too slowly, the drug builds up in the patient’s system and causes severe side effects. Other people can have genetic variants that cause them to have severe side effects to the drug that are unrelated to the rate of drug metabolism.
DNA variants in many different genes have been associated with how the human body responds to over one hundred drugs. Such pharmacogenomic information can help doctors predict whether certain medications or doses will be effective for their patient, or if they are likely to have an adverse reaction to that drug. For example, DNA variants can show that you need a higher or lower dose of a certain drug or that a different drug should be used instead.
Carrier status
 Clinical genome testing can also help people understand how their genes may affect their future family. Many babies born with genetic disorders have parents with no known family history of the disease. In some cases, the babies have a recessive disorder that requires two copies of a disease gene to manifest—one inherited from the mom and one inherited from the dad. The parents are called carriers of the recessive disease since they only have one copy of the disease gene and therefore do not have symptoms of the disease.
Clinical genome testing can also help people understand how their genes may affect their future family. Many babies born with genetic disorders have parents with no known family history of the disease. In some cases, the babies have a recessive disorder that requires two copies of a disease gene to manifest—one inherited from the mom and one inherited from the dad. The parents are called carriers of the recessive disease since they only have one copy of the disease gene and therefore do not have symptoms of the disease.
By analyzing a person’s whole genome, physicians and geneticists can determine if an otherwise healthy person is a carrier of a recessive genetic disease. This can provide individuals with actionable knowledge about their reproductive risk and their chances of having a child with a genetic disease. Carrier screening is for anyone who would like to understand their risk of having a child with a genetic disorder.
How are scientists advancing elective clinical genome sequencing?
The Smith Family Clinic for Genomic Medicine, LLC., located on the campus of the HudsonAlpha Institute for Biotechnology is one of several centers around the United States that offers elective genome sequencing. The team, led by medical director Dr. David Bick and genetic counselor Meagan Cochran, MS, CGC, recently published their findings from 52 participants in the elective genome sequencing program.
Before having their genomes sequenced, participants met with Dr. Bick and a genetic counselor to review their personal health information, family history, and physical exam results. The participants were also counseled regarding potential outcomes and result types, benefits and limitations of testing, and other considerations for testing. Afterwards, they provided a blood sample for the genome sequencing and a saliva sample for the pharmacogenomic panel.
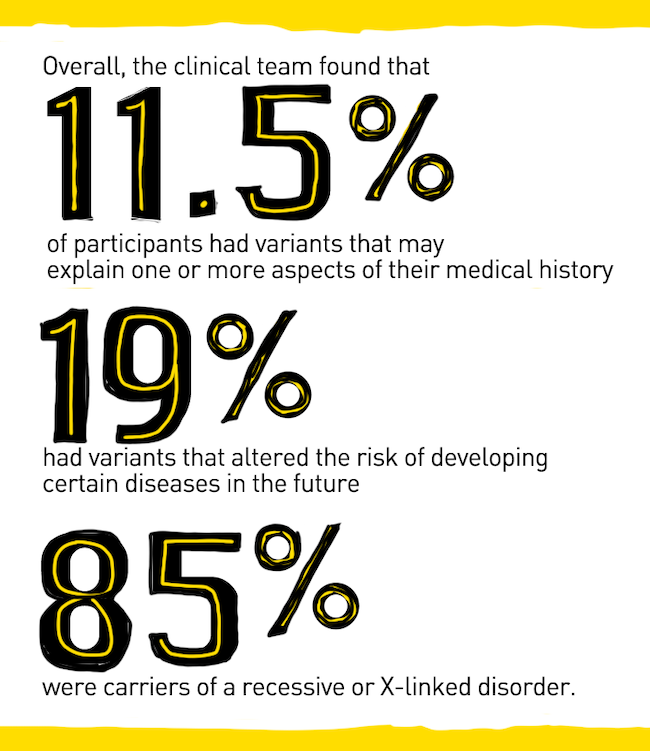
Overall, the clinical team found that 11.5 percent of participants had variants that may explain one or more aspects of their medical history, 19 percent had variants that altered the risk of developing certain diseases in the future, and 85 percent were carriers of a recessive or X-linked disorder. Through the separate pharmacogenomic panel the team found that 100 percent of the participants had pharmacogenetic variants that affected current and/or future medications.
The study shows the value of elective genetic testing when the participant’s medical and family history are incorporated into the analysis of their genomes. Although there is still much progress to be made in elective genome sequencing, this study highlights how elective genome sequencing allows individuals to realize the promise of personalized medicine.


